How an exonuclease decides where to stop in trimming of nucleic acids: crystal structures of RNase T–product complexes Yu-Yuan Hsiao1, Yulander Duh1,2, Yi-Ping Chen1, Yi-Ting Wang1 and Hanna S. Yuan1,* 1. Institute of Molecular Biology, Academia Sinica, Taipei, 11529 and 2, Department of Biotechnology and Laboratory Science in Medicine, National Yang Ming University, Taipei, 11221, Taiwan, ROC Received April 11, 2012; Revised May 14, 2012; Accepted May 15, 2012 |
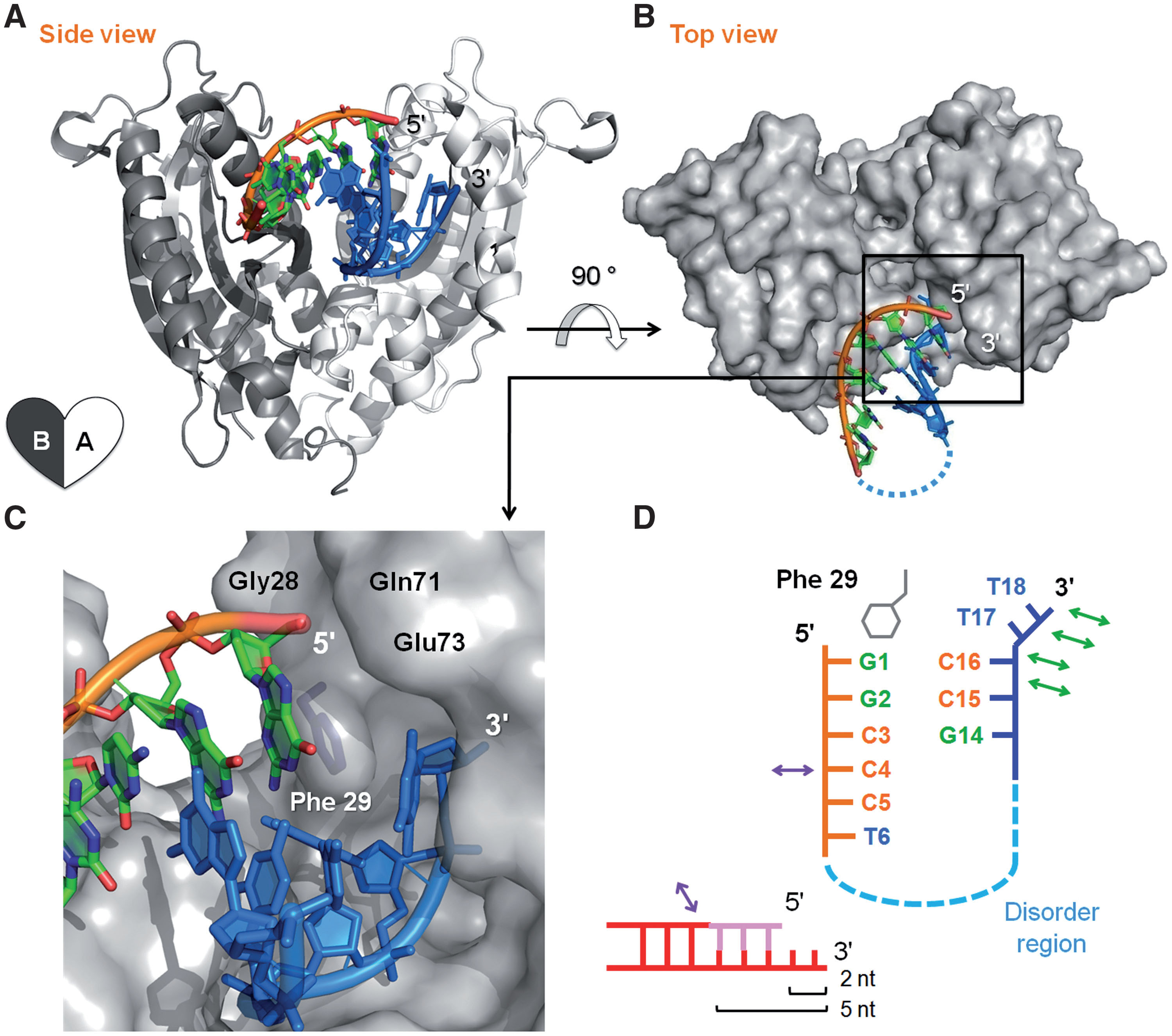
| ABSTRACT Exonucleases are key enzymes in the maintenance of genome stability, processing of immature RNA precursors and degradation of unnecessary nucleic acids. However, it remains unclear how exonucleases digest nucleic acids to generate correct end products for next-step processing. Here we show how the exonuclease RNase T stops its trimming precisely. The crystal structures of RNase T in complex with a stem-loop DNA, a GG dinucleotide and single-stranded DNA with different 30-end sequences demonstrate why a duplex with a short 30-overhang, a dinucleotide and a ssDNA with a 30-end C cannot be further digested by RNase T. Several hydrophobic residues in RNase T change their conformation upon substrate binding and induce an active or inactive conformation in the active site that construct a precise machine to determine which substrate should be digested based on its sequence, length and structure. These studies thus provide mechanistic insights into how RNase T prevents over digestion of its various substrates, and the results can be extrapolated to the thousands of members of the DEDDh family of exonucleases. |
 |
| pdb |