|
The crystal structure of the ImmE7
protein suggests a possible colicin-interacting surface.
Chak, K.-F., Safo, M. K., Ku, W.-Y., Hsieh, S.-Y., and
Yuan, H. S. Abstract 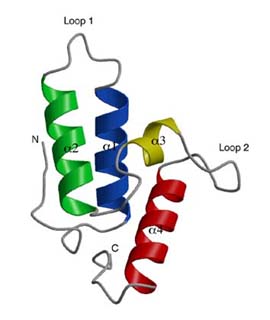 The immunity protein of colicin E7 (ImmE7) can bind specifically to the
DNase-type colicin E7 (ColE7) and inhibit its bactericidal activity.
Here we report the 1.8 ?crystal structure of the ImmE7 protein. This is the first X-ray structure determined in the superfamily of colicin
immunity proteins. The ImmE7 protein consists of four antiparallel
a-helices, folded in a topology similar to the architecture of a four-helix
bundle structure. A region rich in acidic residues is identified. This negatively charged area has the greatest variability within the family
of DNase-type immunity proteins; thus it seems likely that this area is
involved in specific binding to colicin. Based on structural, genetic
and kinetic data, we suggest that all the DNase-type immunity proteins,
as well as colicins, share a "homologous-structural framework" and that
specific interaction between a colicin and its cognate immunity protein
relies upon how well these two proteins' charged residues match on the
interaction surface, thus leading to specific immunity of the colicin.
The immunity protein of colicin E7 (ImmE7) can bind specifically to the
DNase-type colicin E7 (ColE7) and inhibit its bactericidal activity.
Here we report the 1.8 ?crystal structure of the ImmE7 protein. This is the first X-ray structure determined in the superfamily of colicin
immunity proteins. The ImmE7 protein consists of four antiparallel
a-helices, folded in a topology similar to the architecture of a four-helix
bundle structure. A region rich in acidic residues is identified. This negatively charged area has the greatest variability within the family
of DNase-type immunity proteins; thus it seems likely that this area is
involved in specific binding to colicin. Based on structural, genetic
and kinetic data, we suggest that all the DNase-type immunity proteins,
as well as colicins, share a "homologous-structural framework" and that
specific interaction between a colicin and its cognate immunity protein
relies upon how well these two proteins' charged residues match on the
interaction surface, thus leading to specific immunity of the colicin.
Proc. Natl. Acad. Sci. USA, 93, 6437-6442 (1996). |