|
The Transactivation Region of the FIS
Protein that Controls Site-Specific DNA Inversion Contains Extended Mobile
Beta-Hairpin Arms
Safo, M. K., Yang, W. Z., Corselli,
L., Cramton, S. E., Yuan, H. S., Johnson, R. C.
Abstract
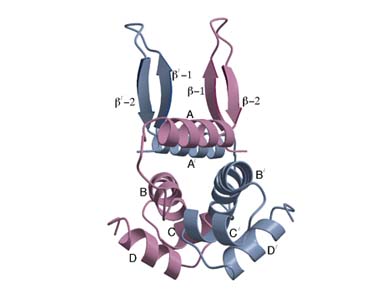
Fis is an Escherichia
coli site-specific DNA-binding protein that functions as a regulator of
many different reactions. X-ray crystal structure analyses have been carried
out for wild-type Fis. The C-terminal of Fis comprises a helix-turn-helix
DNA binding motif (C and D helices), however, the N-terminus of Fis, sequence
1 to 25, that is required to promote in-mediated DNA inversion, is not
resolved in the crystal structure. A new crystal form of a mutant Fis protein
Glu36-Lys now reveals that the trans-activation region contains two beta-hairpin
arms that protrude over 20 Angstrom from the protein core. The newly resolved
beta-hairpin arm at the Fis N-terminal region could be responsible for
interacting with invertases.
The EMBO Journal 16:6860-6873,
1997 |
