| A Novel Role of Imme7 in the
Autoregulatory Expression of the Cole7 Operon and Identification of Possible
Rnase Active Sites in the Crystal Structure of Dimeric Imme7
Hsieh, S. Y., Ko, T. P., Tseng, M. Y., Ku, W. Y., Chak, K. F., Yuan, H. S 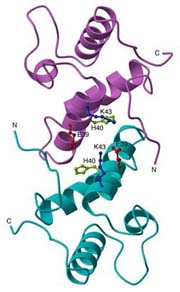
Abstract ImmE7 was originally identified as an inhibitor of the DNase activity of ColE7 in Escherichia coli and recently ImmE7 was found to function as a ribonuclease which specifically cleaves its own messenger RNA. The three-dimensional structure of dimeric ImmE7 has been determined at 1.8 Angstrom resolution by X-ray crystallographic analysis. The dimeric structure is stabilized by the hydrophobic interactions mediated by the sidechains from two counter alpha helices and the N-terminal strand which is oriented in an open conformation to interlock the opposing subunit. The structure of the dimeric ImmE7 presents possible ribonuclease active sites similar to those of mammalian RNase A or microbial barnase. Several residues, including Glu-39, His-40 and Lys-43, possibly are involved in the phosphodiester hydrolysis reaction. The proposed composite active sites are located in the dimeric interface, thus dimeric ImmE7 could represent a novel class of ribonuclease.
|