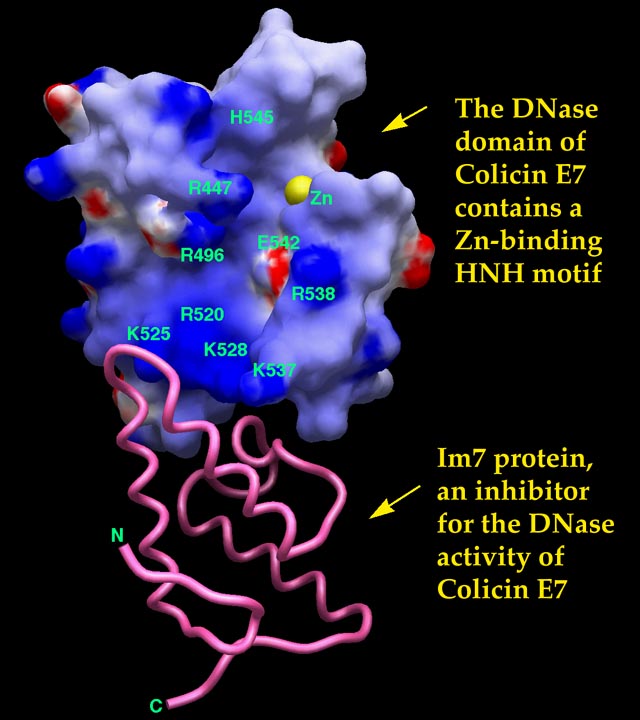
| The crystal structure of the Dnase
domain of colicin E7 in complex with its inhibitor Im7 protein
Tzu-Ping Ko, Chen-Chung Liao, Wen-Yen
Ku, Kin-Fu Chak and Hanna S Yuan
Results: The crystal structure of a one-to-one complex between the DNase domain of colicin E7 and its cognate immunity protein Im7 has been determined at 2.3 Å resolution. The structure of Im7 in the complex is a varied four-helix bundle identical to the previously determined free Im7. The structure of the DNase domain of ColE7 displays a novel a/b fold and contains a zinc ion bound to three histidine residues and one water molecule in a distorted tetrahedron geometry. Im7 has a V-shaped structure extending two arms to clamp the DNase domain of ColE7. One arm (a1*-loop12- a2*) is located in the most varied region of the immunity protein subfamily and this arm primarily uses acidic side chains to interact with the basic side chains in the DNase domain of ColE7. The other arm (loop23-a3*-loop34) is more conserved and it interacts not only with the side-chain but also with the main-chain atoms of the DNase domain of ColE7. Conclusions: The protein interfaces between the DNase domain of ColE7 and Im7 are charge-complementary and the protein complex is predominantly stabilized by charge interactions. The more varied arm in Im7 dominates the binding specificity of the immunity protein to its cognate colicin. Both biological and structural data suggest that the DNase active site for ColE7 is probably near the metal-binding site. Structure, 7:91-102, 1999 |