 |
|
Our
Research Interest
We investigate RNA- and DNA-binding proteins that play key roles in nucleic acids metabolism. The overall goal is to discover the structure-based mechanism of these proteins in nucleic acids recognition, processing, modification and degradation. Using the main tool of X-ray crystallography, together with biochemical and biophysical approaches, we have shown how a group of proteins function in trimming, unwinding, degradation and modification of RNA/DNA in diverse ways. Over expression, mutations and misfolding of most of the proteins that we study are linked to human diseases, ranging from neurodegenerative disorders to inflammatory diseases. Therefore, understanding how these proteins recognize RNA/DNA and participate in nucleic acids metabolism and how they lose their functions not only advance our knowledge in the working mechanisms of RNA/DNA metabolism, but also pave the way for strategy development of disease treatments. The brief summary of two major projects is listed below. 1.Structural investigation of mitochondrial RNA decay and DNA maintenance Mitochondria are the power plants that provide the energy source for all the cellular processes and therefore mitochondria dysfunctions are frequently associated with pathologic conditions, including aging and inflammation. However, our understanding for the regulation and maintenance of RNA and DNA levels in mitochondria are limited. We focus on mitochondrial enzymes to unveil the molecular mechanism of mitochondrial RNA decay and DNA maintenance. In human mitochondria, we showed how long-chain RNAs are degraded cooperatively by PNPase and Suv3 helicase, while short nanoRNAs are specifically targeted by Rexo2. We also identified the unique function of ExoG in cleaving at the junction between RNA and DNA for removal of RNA primers during mitochondrial DNA replication,and the link between EndoG's activity and oxidative stress-induced mitochondria dysfunction. 2.Structural insights into DNA modification by DNA methyltransferases DNA methylation is a key mechanism of epigenetic regulation established and maintained by DNA methyltransferases (DNMTs) in mammals. The de novo DNA methyltransferases, DNMT3A and DNMT3B, are the primary enzymes that methylate cytosines in CpG sites to create DNA methylation patterns during embryogenesis and establish genomic imprints during germ cell development. We study how human DNMT3B recognizes and methylates CpG sites and how the activity of DNMT3B is allosterically regulated by chromatin modifications, chromatin proteins and enzyme post-translational modifications by biochemical and structural approaches. Moreover, as hypermethylation of tumor suppressor genes and overexpression of DNMTs have been established as the major key players in cancer, DNMT inhibitors are promising anticancer drugs. We also investigate the activities of DNMT inhibitors and their inhibition mechanisms to provide a basis for developing new DNMT inhibitors. |
Structure Gallery   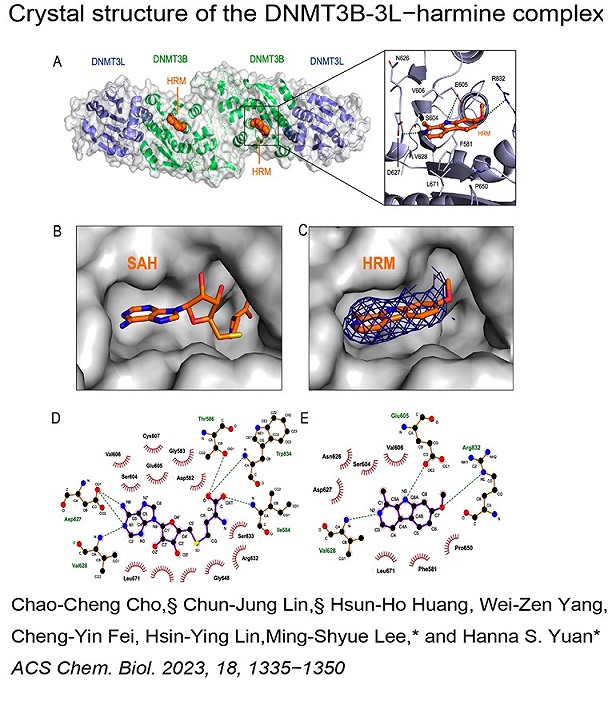 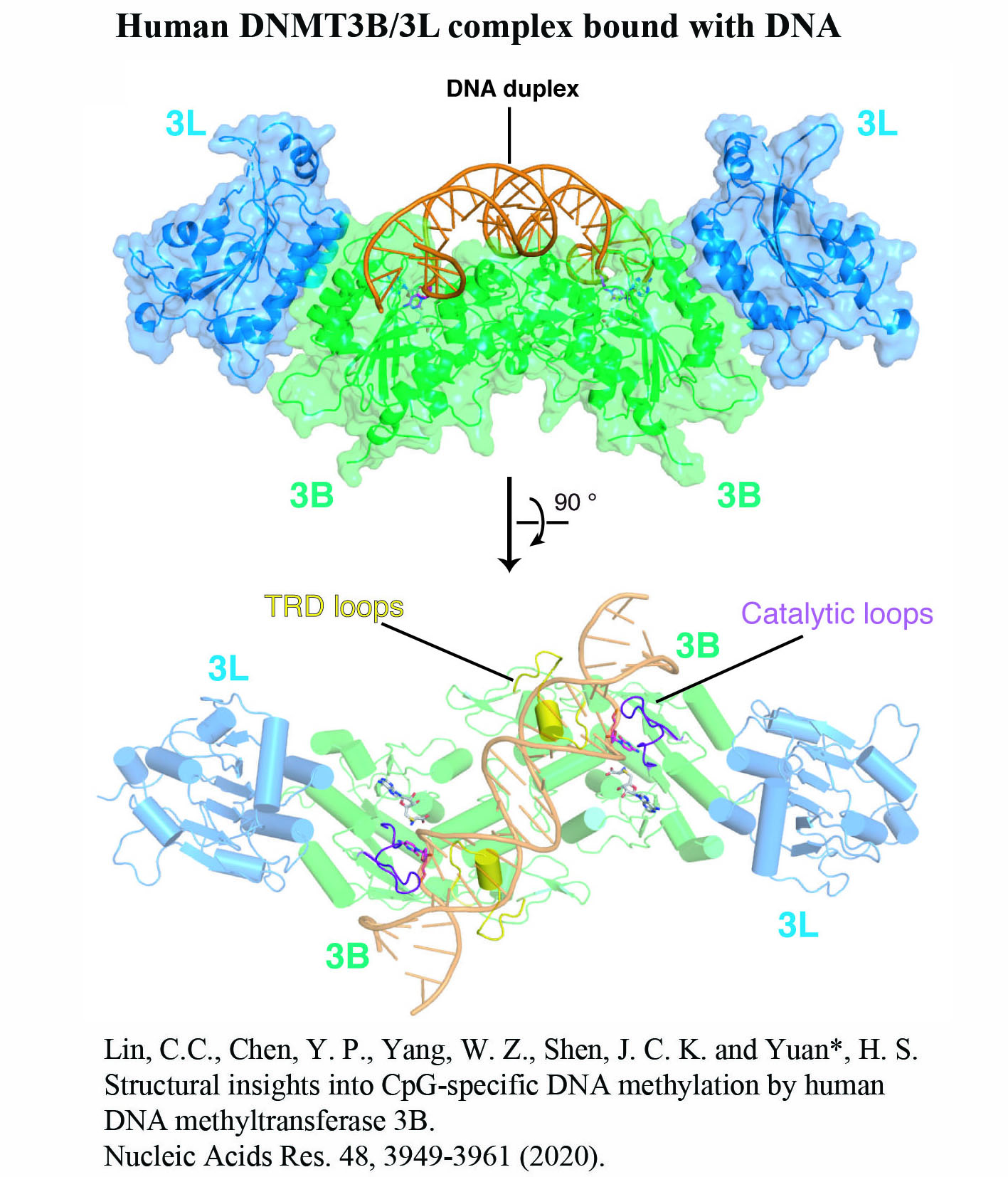 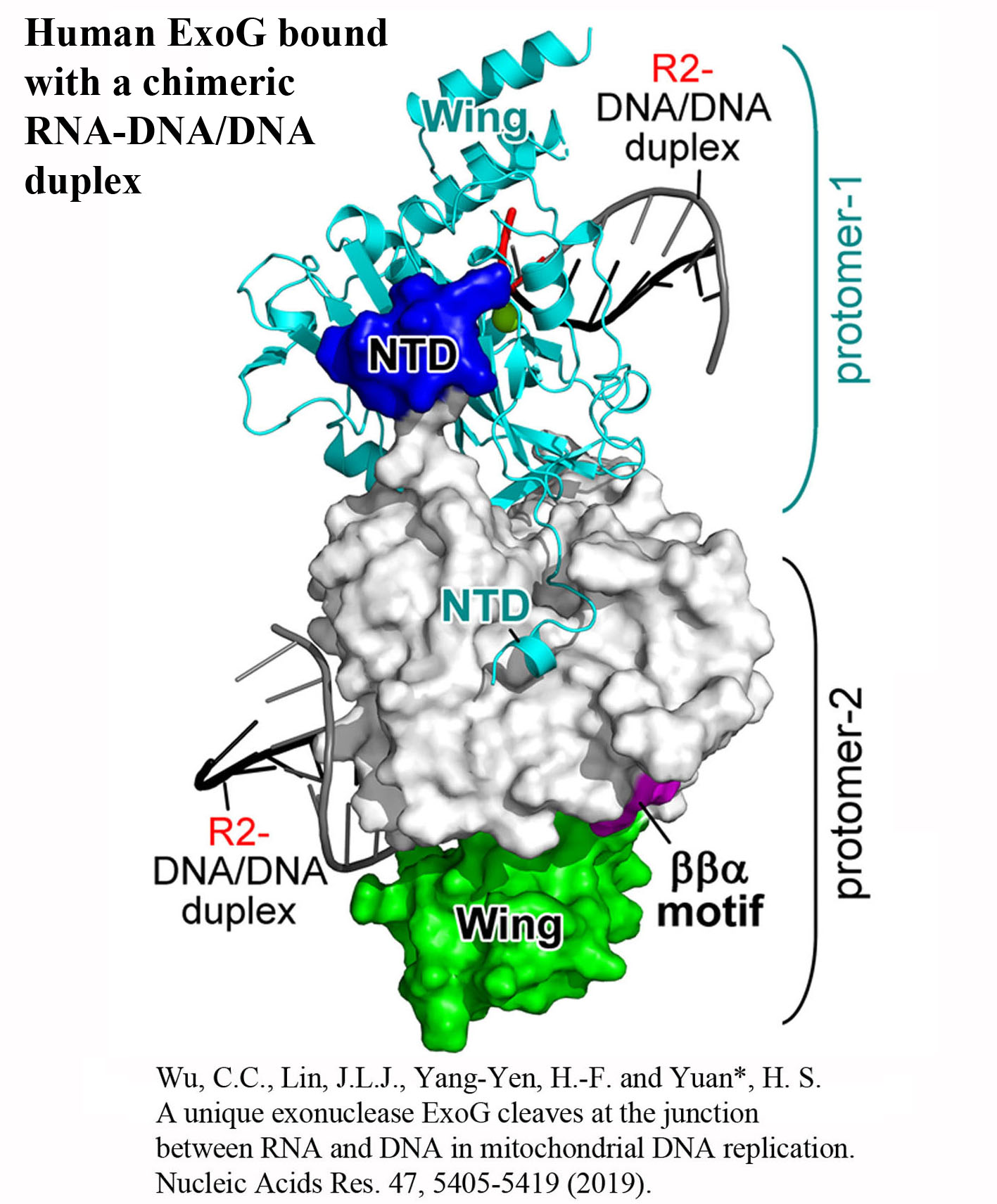 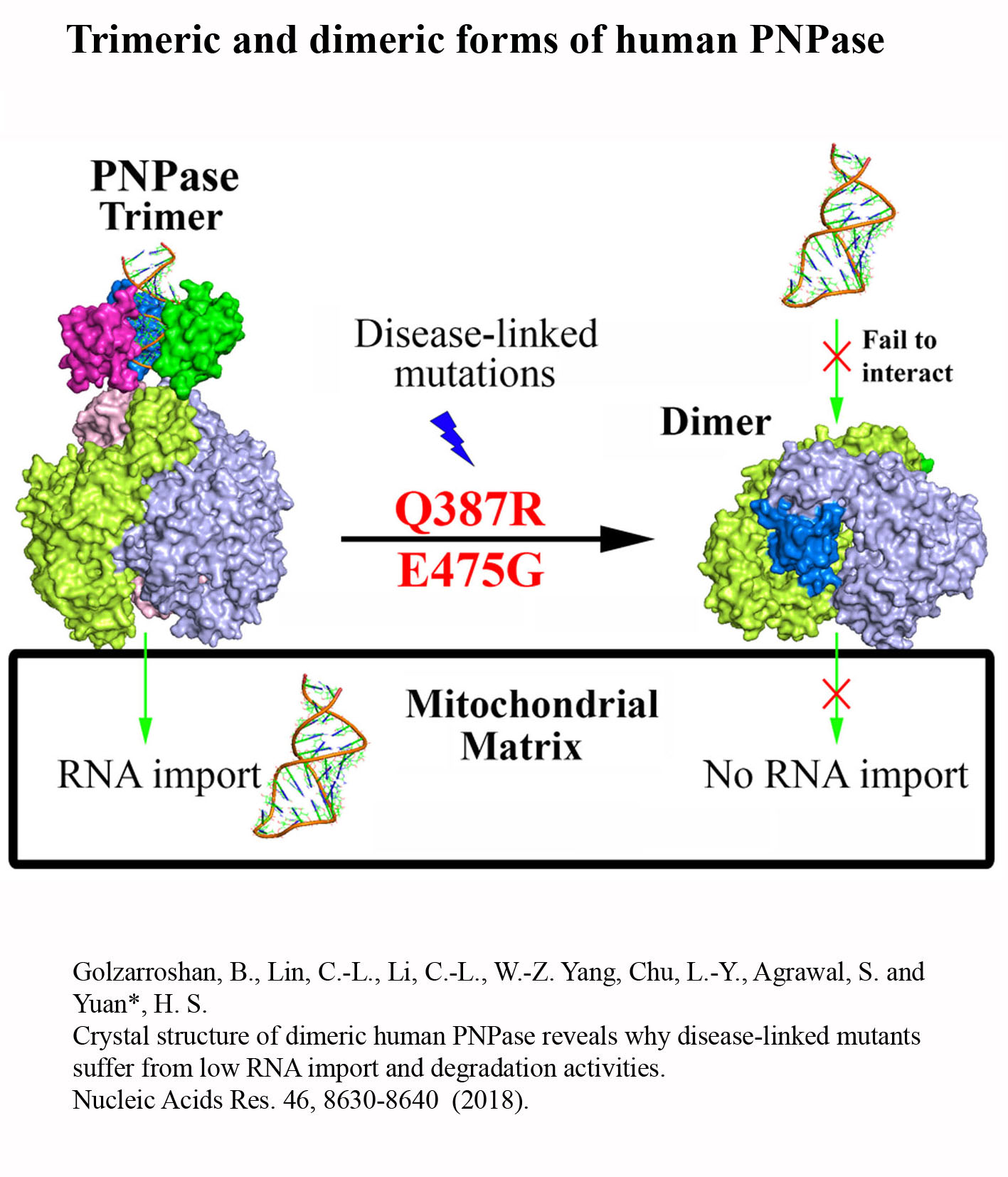 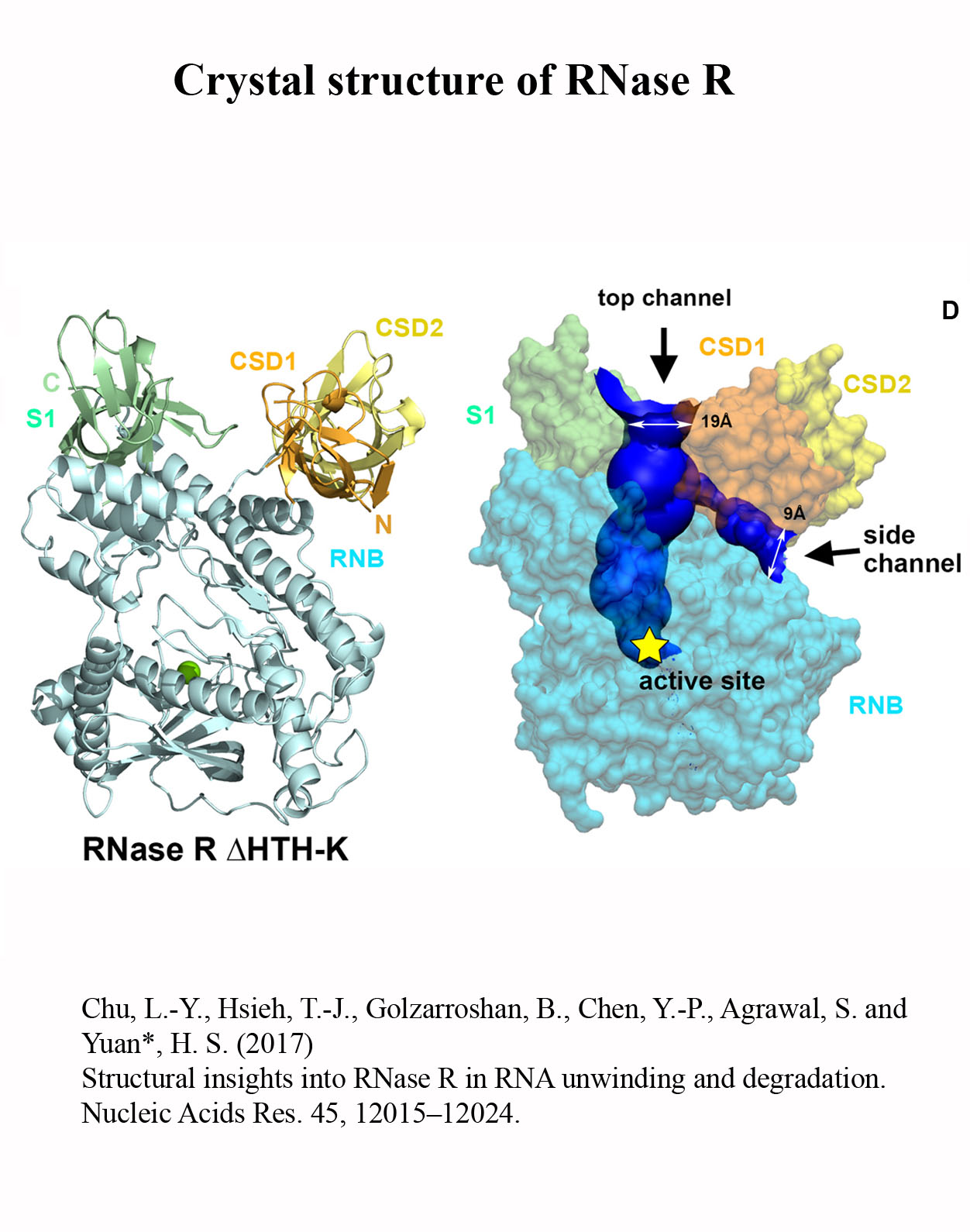 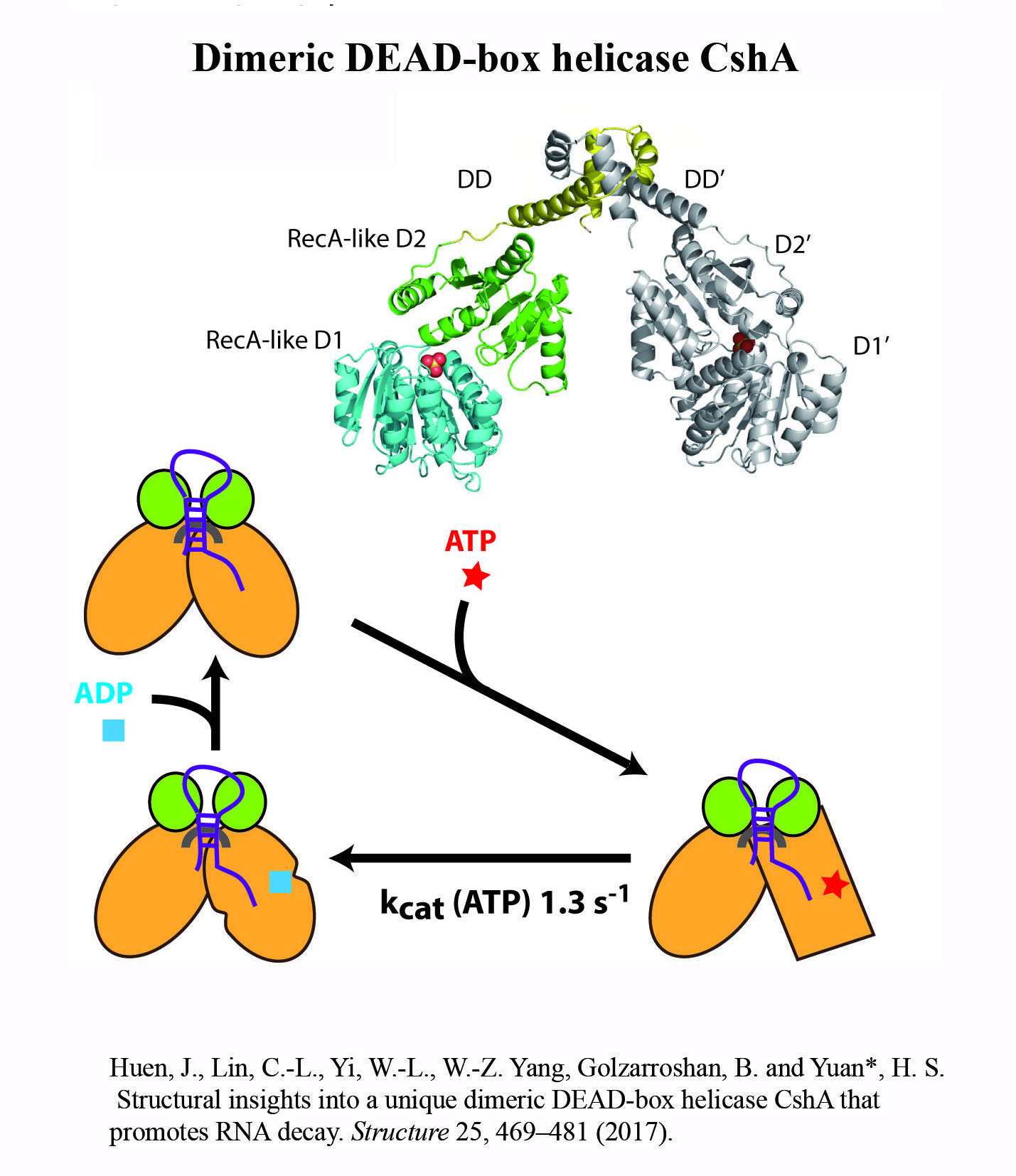 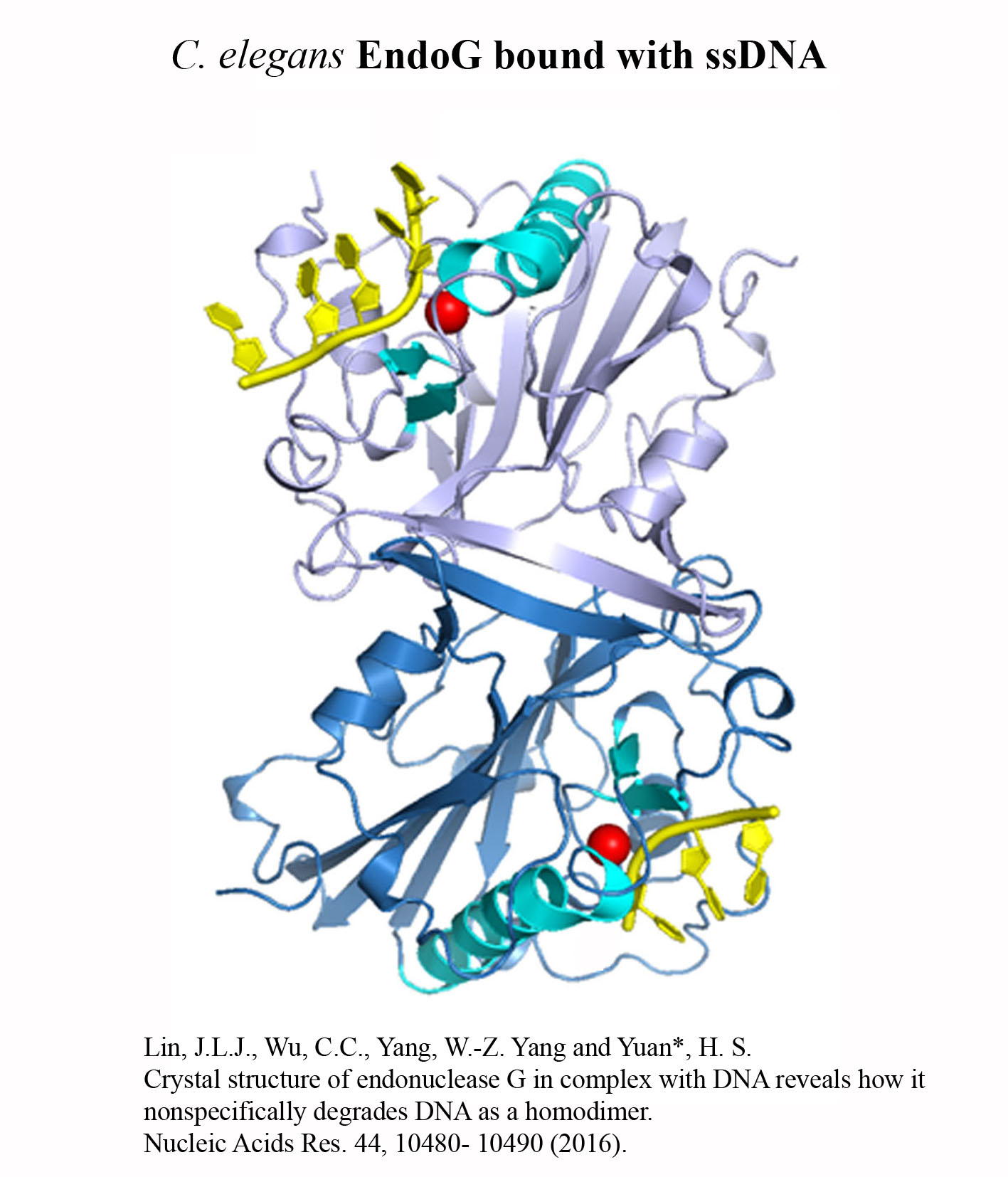 研究方向 我們研究在核糖核酸代謝中,扮演關鍵角色的RNA和DNA結合蛋白質。總體目標是闡明這些蛋白質在核糖核酸代謝中的分子機制。我們主要運用X光晶體學的方
法,來測定蛋白質與核糖核酸結合的晶體三維結構,配合生物化學和生物物理學方法,來研究這些蛋白質,如何以不同的方式展開在RNA
和DNA上的辨識、加工、降解和修飾的作用機制。我們研究的大多數蛋白質的突變、數量改變或錯誤折疊,多與人類疾病相關,包括神經退行性疾病、發炎性和粒
線體疾病。因此了解這些蛋白質如何識別核糖核酸並參與核糖核酸代謝,以及它們如何喪失功能,不僅可以增進我們對核糖核酸代謝機制的了解,也在疾病機制和治
療上,建立不可或缺的基礎。
|
 |